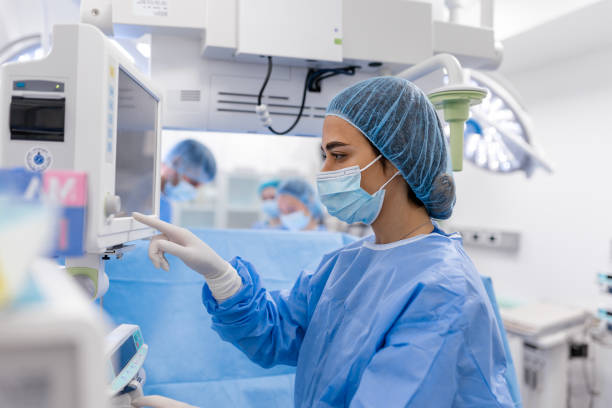
Medical Devices Combination Products
Contact us to the email
Mail Anytime
support@jeffyuen.com
Medical Devices and Combination products present unique challenges both in the science of their development and commercialization and the in the regulations with which you need to comply. JYA’s experts know how to navigate this landscape and know the technical challenges you face. Let our team of experts help you solve these problems.
Our support includes Class I, II and III Medical Devices and Combination Products (including Biologics and Software). We can also support your 510k PMA activities
Wealth Management
Audit Marketing
Finance Consulting
Banking Advising
Cost
of Work
Suspendisse finibus urna mauris, vitae consequat quam vel. Vestibulum leo ligula, vit commodo nisl Sed luctus venenatis pellentesque.
Suspendisse finibus urna mauris, vitae consequat quam vel. Vestibulum leo ligula, vit commodo nisl Sed luctus venenatis pellentesque.
Suspendisse finibus urna mauris, vitae consequat quam vel. Vestibulum leo ligula, vit commodo nisl Sed luctus venenatis pellentesque.
Suspendisse nec urna nec tellus vulputate. At enim trud exercitation dolor ullamco laboris nisut aliquip aute irure dolor in reprehenderit.
Contact Experts

- Nsectetur cing elit.
- Suspe susc sagittis leo.
- Entum dignissim posuere.
Experts Associate

Bob Mehta
Sr Associate
Bob Mehta
Sr Associate


Mhashid Zahed
Sr Associate
Mhashid Zahed
Sr Associate


Susan Needle
Sr Associate
Susan Needle
Sr Associate


Trent Davalos
Sr Associate
Trent Davalos
Sr Associate


Moj Eram
Sr Associate
Moj Eram
Sr Associate


Peter Knauer
Combination Product - CMC/RA
Peter Knauer
Combination Product - CMC/RA


Laurie Auerbach
Sr Associate
Laurie Auerbach
Sr Associate